Question: What is the last resort in stain removal?
Answer: Bleaches (no, we are not referring to scissors).
Bleaching agents can be used as an effective tool in the stain removal process. Bleaches should be used after limited success with various dryside and wetside agents. Also, some stains will be lightened with normal stain removal procedures, but may require the use of a bleach to remove the last traces of a stain. Some examples of these stains may include ink and dye transfer.
Bleaches do not remove staining material. They simply render them invisible by either adding oxygen or taking oxygen away. It is safer for the fabric and color if stains are removed using normal stain removal procedures. This is why bleaching is done as a last resort.
TYPES OF BLEACHES
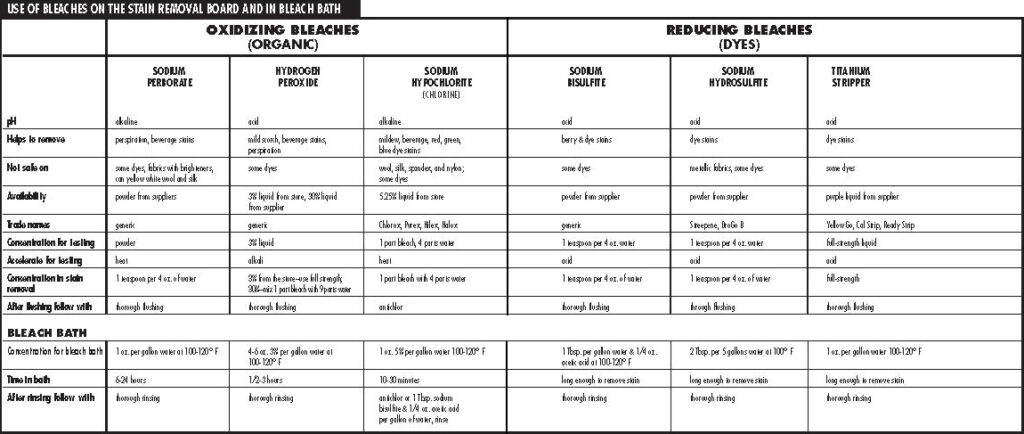
There are two categories of bleaches–oxidizing and reducing bleaches. Oxidizing bleaches make stains “colorless” by adding oxygen. These bleaches work best on organic stains, which would include foods, beverages, plant-based stains, perspiration, and body discharges. The following is a list of commonly used oxidizing bleaches from weakest strength to strongest.
- Sodium perborate
- Hydrogen peroxide
- Sodium hypochlorite (chlorine)
Reducing bleaches remove or take away oxygen from stains to make them appear “colorless.” These bleaches are most effective on dye stains. The following is a list of commonly used reducing bleaches from weakest strength to strongest.
- Sodium bisulfite
- Sodium hydrosulfite
- Titanium stripper
GENERAL PRECAUTIONS
There are numerous precautions that must be followed when using bleaches in stain removal. However, the most important precaution is to test for colorfastness. This process may be done on the garment (very risky) or on a small sample of fabric clipped from the seam allowance. Test all colors that will come in contact with the bleach.
Other precautions to be followed when using bleach include:
- Always wet area with water before applying bleach. This:
- removes traces of previous stain removal agents.
- ensures fabric is in a neutral state.
- breaks surface tension, allowing bleach to penetrate into fabric.
- Work over a towel.
- Do not let bleaches contact metals because they can cause color change.
- Accelerate bleaching action only when testing, which will let you know the degree of color loss that may occur.
- Do not tamp.
- Use an eyedropper with liquid bleaches or spotting bottle with a controlled drip top.
- NEVER pour directly from original bottle.
PRECAUTIONS FOR BLEACH BATHS
• Garment must be cleaned before bleaching.
• When practical, remove metal fasteners and pins.
• Dissolve bleach thoroughly in water before placing item in bleach bath.
• Keep completely immersed.
• Use plastic or nonreactive containers (no metal tubs or containers).
• Thoroughly rinse and/or neutralize after performing a bleach bath.
HOW TO CHOOSE WHICH BLEACH TO USE
Selecting which bleach to use is not as easy as it seems. First, you must try to identify the type of stain on the item. This will help you determine which category of bleach (oxidizing or reducing) to use. Also, identify the fiber type, which may limit the bleaches that can be safely used. Next, you must test for colorfastness. Always start with the weakest and safest bleach for the fabric, and then progress, if necessary, to the strongest. Make sure to test all colors in the affected areas. If the bleach is safe for the color and fiber type, the next step would be to test it on the stain to see if it will remove it. This process should be followed for all bleaches and on all stains present on the item. Remember, bleaches that will effectively remove perspiration and beverage stains may not be as effective on ink and dye stains.
The following is an overview of the commonly used oxidizing and reducing bleaches. Special precautions, if any, will also be listed. For bleach bath procedures, see chart below.
Oxidizing Bleaches
Sodium Perborate (Alkaline)
- works best on: perspiration and beverage stains
- where to purchase: supplier
Sodium perborate, commonly referred to as “perborate,” is the weakest oxidizing bleach. This bleach is safe for all fibers, but is not safe on all colors. Due to the presence of fluorescent brightening agents on silk and wool, these fabrics may yellow or discolor if perborate is used.
To use in stain removal, mix one teaspoon of the perborate powder with four ounces of water. Due to loss of effectiveness of the solution when mixed with water, a new solution should be made everyday. When testing, apply the bleach powder to the wet fabric. Use a synthetic detergent to saturate the powder. Accelerate with heat. Heat makes the bleach more active. To obtain this heat when testing, use the steam-air gun in a “fogging” operation. Partially depress the steam gun pedal to produce a small “fog” or “curl” of steam from the nozzle. Hold the gun 1/4 inch from the fabric to fog. After fogging, carefully observe the sample to see if any discoloration has occurred. This should be done when the fabric is wet, and after it has dried completely. If tests show no discoloration, this bleach may be safely used on the item.
In most instances, thorough rinsing will remove all traces of the bleach. However, since perborate is alkaline, it may be beneficial to neutralize the area with an acid, especially on silk and wool.
Hydrogen Peroxide (Acid)
- works best on: mild scorch, perspiration and body discharges, beverage stains, old stains
- where to purchase: supplier, grocery stores, drug stores
Hydrogen peroxide, commonly referred to as “peroxide,” is available from suppliers, as well as in grocery and drug stores. If purchased from a supplier at full strength of 30 percent, it should be diluted down to 3 percent. This can be done by mixing one part full-strength peroxide with nine parts of water. Peroxide purchased from grocery and drug stores is sold at the recommended 3 percent for safe stain removal.
As with all bleaches, test for colorfastness before using peroxide. To test, clip a sample from an unexposed seam area, wet the fabric, and place a few drops of peroxide on the sample. Next, apply a few drops of ammonia (26°Baume) to accelerate for testing. This allows you to determine the potential for causing discoloration. In actual use, you should only apply the bleach, and should not accelerate for color and fabric safety.
Peroxide is a slow-acting bleach, so you must have patience when using this bleach. In most cases, once the bleach is applied, you should set it aside for 10 to 15 minutes. Observe to see if the stain has been removed and re-apply if necessary. After using, flush the area thoroughly.
Sodium Hypochlorite (Alkaline)
- works best on: beverage stains, dye stains
- where to purchase: grocery stores, drug stores
Sodium hypochlorite, commonly referred to as “chlorine,” is used in a liquid form. This bleach is sold in grocery and drug stores at 5 percent. Common trade names include Chlorox, Lintex, Purex, Hilex, and Halox. When using for stain removal, this bleach should be diluted to 1 percent (one part bleach to four parts water). This bleach is not safe on all colors or fibers. Always test for colorfastness before using this bleach. Testing can be performed by clipping a sample from the seam allowance, wetting the fabric, and applying a few drops of bleach. Accelerate the bleaching action with heat during testing.
In addition to causing discoloration, chlorine bleach may contribute to fabric weakening and damage on cellulose fibers, such as cotton, linen, rayon, and ramie. After using, the area should be flushed thoroughly, and neutralized with an anti-chlor. Anti-chlors may be purchased pre-mixed from your supplier or made by mixing sodium bisulfite, acetic acid, and water (see chart on previous page).
Chlorine bleach should not be used on silk and wool. Also, nylon fibers may yellow, and spandex fibers may deteriorate with chlorine bleach.
Reducing Bleaches
Sodium Bisulfite (Acid)
- works best on: berry stains, iodine
- where to purchase: supplier
The weakest reducing bleach is sodium bisulfite. This white powder bleach is commonly referred to as “bisulfite.” To use, mix one teaspoon of the bisulfite powder with four ounces of water. Due to loss of effectiveness of the solution when mixed with water, a new solution should be made everyday.
When testing, clip a sample from the seam allowance, wet the sample, and apply the bleach. To accelerate in testing, use 28% acetic acid. When finished, flush thoroughly to remove all traces of the bleach. If the fabric is left in an acid condition after use, fabric weakness and degradation may occur.
When used in the recommended concentration, bisulfite is safe on all fibers and most colors. However, its main use is in anti-chlors, which are used to remove the last traces of chlorine bleach.
Sodium Hydrosulfite (Acid)
- works best on: dye stains
- where to purchase: supplier
This bleach is sold as a white powder, and is commonly referred to as “hydro.” To use, mix one teaspoon of powder with four ounces of water. Since the powder bleach loses its effectiveness when mixed with water, a new solution must be made everyday. To test for colorfastness, wet the fabric, apply the bleach solution, and a few drops of 28 percent acetic acid to accelerate. The bleach should only be accelerated for testing, and not when using on the actual fabric/garment.
After use, thoroughly rinse from the fabric to prevent weakening of the fabric from the acid bleaching agent.
This bleach is primarily used to remove dye stains. Also, this bleach can often make whites appear whiter. Hydro is not safe on metallic fibers. Also, do not let hydro make contact with metal surfaces or objects since it can turn the fabric black.
Titanium Stripper (Acid)
- works best on: dye stains
- where to purchase: supplier
Titanium stripper, which is the strongest reducing bleach, is purchased through a supplier in a dark violet liquid form. This bleach, which is commonly referred to as “stripper,” is used full strength. It is recommended that the bleach be placed in an eye dropper bottle, or a bottle with a controlled drip top. Titanium stripper is safe for all fibers, but not all colors. To test, wet a sample taken from an unexposed seam, and apply a few drops of the bleach. Fog, as described for use with sodium perborate, the area with steam to accelerate the bleaching action. After using titanium stripper, flush the fabric several times to thoroughly remove all traces of the bleach.
Titanium stripper should be stored in a dark bottle with a secure cap to maximize shelf life. If the liquid crystallizes, it should be discarded.
If titanium stripper is used in areas where an alkali was used, a black stain may appear. This black stain may be removed with acids, such as acetic acid or rust remover. If used in areas where sodium bisulfite or sodium hydrosulfite have not been thoroughly flushed from the fabric, yellowing may occur. Use rust remover to remove the discoloration.
This article is from IFI’s Bulletin IF Nov00.

